Abstract
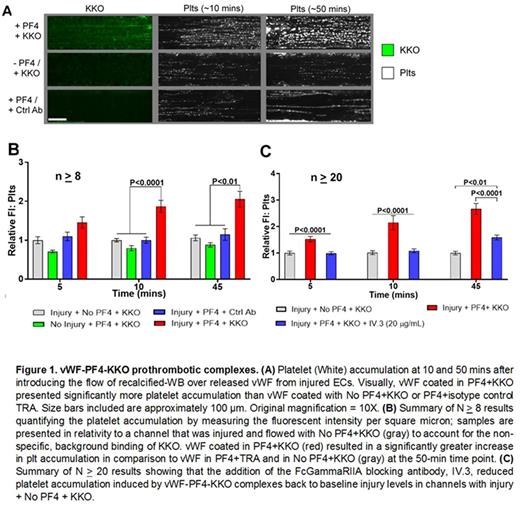
Heparin-induced thrombocytopenia (HIT) is driven by the binding of pathogenic HIT antibodies to platelet factor 4 (PF4) complexed to heparin. We, and others, have shown that PF4 bound to a variety of other polyanionic molecules, like surface glycosaminoglycans, also form HIT antigenic complexes. This is likely due to the formation of high molecular weight aggregates by the PF4. While studying the pathogenesis of HIT in a microfluidic system lined with human umbilical endothelial cells (HUVEC), we noted that PF4 did not bind diffusely to the HUVECs after a hematoporphyrin-induced photochemical injury as one would expect if binding were to the injured surface GAGs, but rather in a linear pattern described previously for von Willebrand Factor (vWF) under flow. We confirmed this by concurrent staining using PF4 and vWF-specific labeled antibodies. We also demonstrated that the HIT-like monoclonal antibody KKO, as well as IgG isolated from patients with likely HIT as determined by clinical assessment and ELISA positivity and positive serotonin release assay, bound to these PF4-vWF complexes. The HIT-like complexes were not uniformly distributed along the vWF, but occurred in a periodic fashion along thicker vWF strands. This suggested that the complexes were composed of multiple molecules of PF4 and vWF forming cross-chained high molecular weight aggregates that induce or enhance binding of HIT antibodies as previously reported for PF4/heparin in solution. We then wanted to identify the potential for therapeutics to intervene with PF4-vWF-KKO complex formation or stability. We tested this by introducing heparin; 2-0,3-0 desulfated heparin (ODSH); ADAMTS13 or n-acetylcysteine into channels post-complex formation. All interventions at therapeutically relevant concentrations were able to significantly remove or disrupt complex formation. When whole human blood + PF4 + KKO were flowed through channels lined with injured endothelium, large platelet aggregates developed within 10 mins along the extended strands of vWF. Significantly fewer platelet aggregates formed in the absence of PF4 or with KKO alone or PF4 plus an isotype control TRA. After 1 hour of flow, aggregates in the PF4 + KKO channels had grown significantly, but had largely disappeared in the other settings. Furthermore, by adding the FcgammaRIIA blocking antibody, IV.3, platelet binding could be reduced to background levels. These studies identify large vWF polymeric strands as a binding site for PF4 and a HIT antigen target. The Fc portions of the bound HIT antibody then contribute to platelet binding to the vWF. We are presently examining whether PF4-vWF-KKO aggregates contribute to thrombotic complications in HIT and defining the vWF domain(s) involved as these targets offer a potential novel intervention target in HIT.
Cines: Amgen: Consultancy; Ionic: Consultancy; Juno: Consultancy; Astellas: Consultancy; T2 Biosystems: Research Funding; Rigel: Consultancy; Syntimmune: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.